
Thomson postulated that atoms consisted of the small, negatively charged electrons he had observed-embedded in a very low-density, positively charged, spherical matrix making up the rest of its mass. More than 100 years after Dalton repopularized the concept of atoms, the first attempt to explain their structure had finally been offered. This led Thomson to propose the first structural model for an atom.Thomson’s measurements led him to a startling conclusion: that electrons were much smaller than atoms. Thomson was able to create isolated beams of pure electrons, measuring their mass, velocity, and charge. Cathode-ray tubes played an important role in the advancement of human understanding of atoms.
/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)

But Dalton had no way to effectively probe the structures of his proposed atoms.It’s the combination of these 2 laws that gave Dalton an airtight argument for the existence of indivisible atoms that come together in these simple whole-number ratios to create molecules.He also found that when 2 elements combine to form more than one compound, the weights of one of the elements that combine with a fixed weight of the other are in a ratio of very simple whole numbers.He found that when 2 elements combine to form a compound, the mass of all the products is equal to the masses of all the starting materials.
/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)
Using relatively simple observations, Dalton was able to formulate a sound atomic theory.What he did have, though, was a keen intellect and the benefit of the work of Antoine Lavoisier and others. Dalton didn’t have the sophisticated scientific instrumentation that we do today, so he had no way to see or experiment directly with atoms. Discourse over the nature of matter and its fundamental units slowed to nearly a halt with the fall of ancient Greece, and it wasn’t until the start of the 19 th century that the concept of the atom was revived, primarily as a result of the work of John Dalton.Nonetheless, the Greek term “atom,” meaning “not divisible,” has stuck and is still how we refer to the smallest quantity of a given element that can exist. Today, we know that none of these are actually elements, and we also know that atoms of true elements can in fact be divided, but that they change to a new element when they are.The Greeks widely believed these elements to be air, water, earth, and fire-and they also believed that particles of these elements were absolutely indivisible. The notion of atoms was first forwarded by ancient Greek philosophers who postulated that there are just a handful of fundamental substances that combine in various ways to form all others.But much smaller, negatively charged electrons orbit this nucleus, balancing out the positive charge provided by the protons. Protons and neutrons are of nearly equal mass and reside in a dense nucleus at the center of the atom.
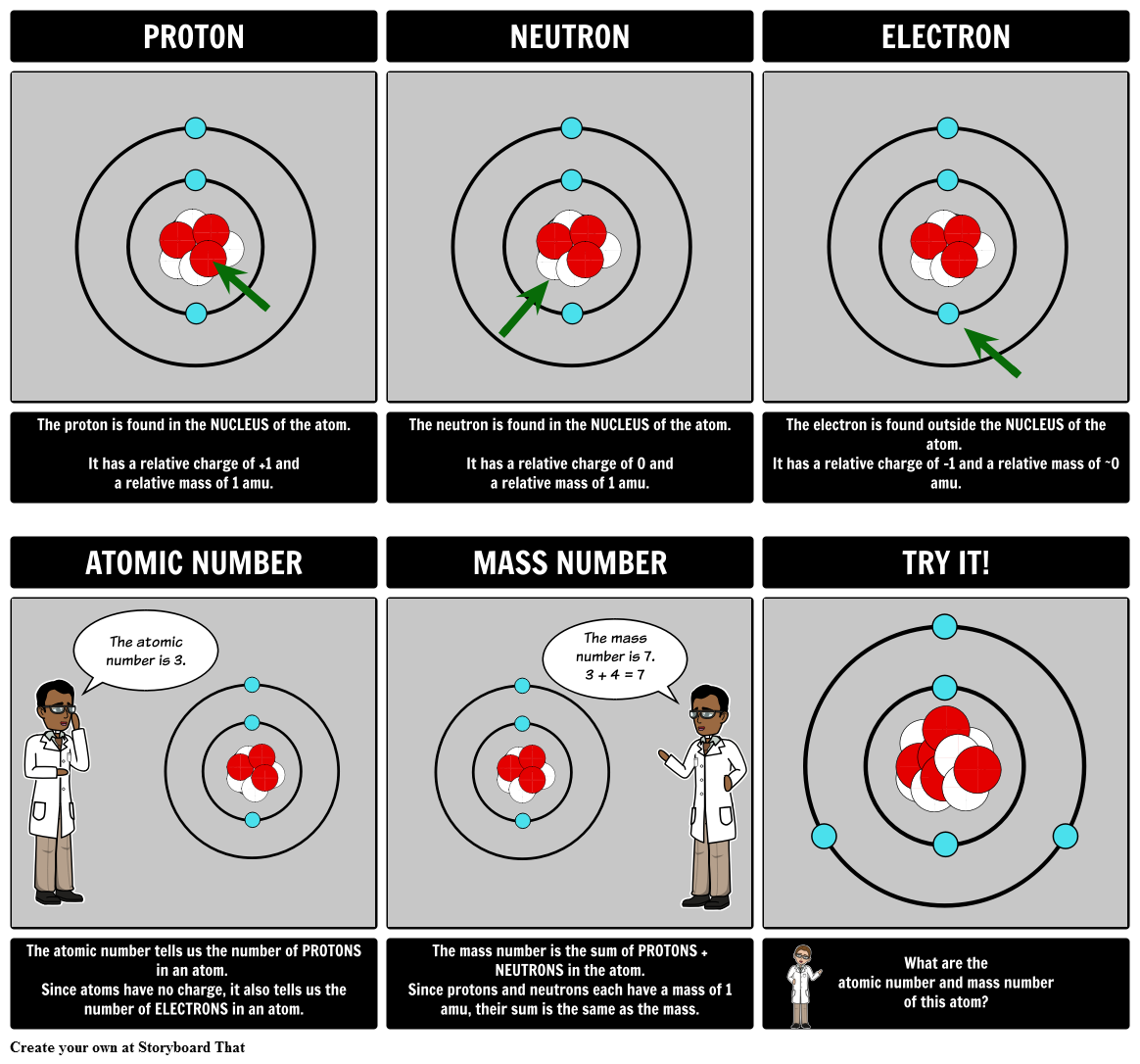
In this lecture, you will learn how each of these particles was discovered and how the culmination of generations of scientific work ultimately came together to create our understanding of the most fundamental unit of matter: the atom.Ītoms are comprised of 3 types of particles called subatomic particles: positively charged protons, negatively charged electrons, and neutrons, which have no charge.


 0 kommentar(er)
0 kommentar(er)
